Abstract
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative treatment option for refractory and relapsed acute myeloid leukemia (R/R AML) in non-remission status. Although human leukocyte antigen (HLA)-matched relatives are the preferred donors for R/R AML patients in non-remission status, their 2-year overall survival following HLA-matched related donor transplantation (MRDT) has only been reported to be 25% due to a high relapse rate of over 50%. Alternatively, cord blood transplantation (CBT) is a donor transplantation procedure that has similar advantages to MRDT in terms of rapid availability, making it possible for urgent HSCT. Moreover, CBT can induce a more potent graft-versus-leukemia (GVL) effect in AML patients under various conditions as compared to other donors. Therefore, this study aimed to investigate the impact of CBT as compared to MRDT using the Transplant Registry Unified Management Program 2 in Japan.
Method
This study included 2451 adult patients with non-remission AML who received CBT (1738 patients) or MRDT (713 patients), including bone marrow transplantation and peripheral blood transplantation, for their first HSCT between January 2009 and December 2018. The primary endpoint was set as 5-year progression-free survival (PFS), and secondary endpoints were set as 5-year overall survival (OS), 5-year cumulative incidence of relapse, 5-year cumulative incidence of non-relapse mortality (NRM), 100-day cumulative incidence of neutrophil engraftment, 1-year cumulative incidence of platelet engraftment, 1-year cumulative incidence of grade II-IV acute graft-versus-host disease (GVHD), and 5-year cumulative incidence of extensive chronic GVHD. The impact of CBT compared to MRDT was then evaluated using propensity score (PS)-matching analysis. In PS calculation, covariates that may influence the selection of the donor and outcomes were selected clinically, and they included the following: age, sex, karyotype risk, disease status, presence of blasts in the peripheral blood, HCT-CI, intensity of conditioning regimen, acute graft versus host disease (GVHD) prophylaxis, and years of HSCT.
Result
A total of 2451 patients, comprising 1738 who received CBT and 713 who received MRDT, were enrolled and analyzed. The median age was 55 years (interquartile range, 43-63 years), and 1484 patients (61%) were male. Differences in the clinical characteristics between the two groups were noted. Specifically, the CBT group had older patients, more patients with relapse, blasts in peripheral blood, a higher hematopoietic cell transplant comorbidity index, and had more patients who received reduced-intensity conditioning regimen, tacrolimus-based acute graft-versus-host disease prophylaxis, and transplanted recently. After PS matching, we selected 918 patients among the 2451 patients receiving CBT or MRD, and the patient characteristics were well balanced between the groups.
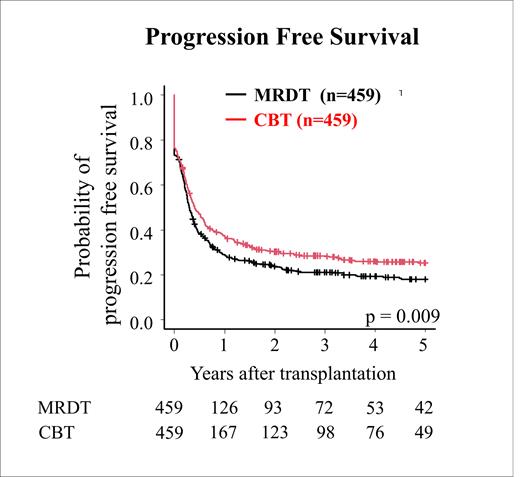
Regarding the primary endpoint, the 5-year PFS was 25.2% (95% CI: 21.1%-25.5%) in the CBT group vs. 18.1% (95% CI: 14.5%-22.0%) in the MRDT group (P=0.009) in the PS-matched cohort (Figure 1). The adjusted hazard ratio (HR) was 0.83 (95% CI: 0.69-1.00, P=0.045).
Regarding secondary endpoints, CBT group compared to MRDT group had similar 5-year OS (28.4% in CBT group vs 21.7% in MRDT group, P=0.152), lower 5-year cumulative incidence of relapse (49.3% s 62.8%, P<0.001), higher cumulative incidence of 5-year NRM (25.5% vs 19.1% P=0.0158), lower 100 days neutrophil engraftment (49.5% vs 93.5%, P<0.001), lower one-year platelet engraftment (62.6% vs 79.1%, P<0.001), similar one-year cumulative incidence of grade II-IV acute GVHD (35.5% vs 30.5%, P=0.092), and lower 5-year cumulative incidence of extensive chronic GVHD (8.6% vs 18.5%, P<0.001) in the PS-matched cohort.
Conclusion
In this population, CBT was associated with a better 5-year PFS as compared to MRDT after allogeneic HSCT.
Kondo: MSD Pharmaceutical: Honoraria; Asahi Kasei Pharmaceutical: Research Funding; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Chugai Pharma: Honoraria; Sumitomo Dainippon Pharma: Honoraria. Kanda: NextGeM Inc: Patents & Royalties; Ono Pharma Inc.: Honoraria; Novartis Pharma K.K.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Sanofi K.K.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; SymBio Pharmaceuticals, Ltd.: Membership on an entity's Board of Directors or advisory committees; Takeda Pharmaceutical Company Limited: Honoraria, Membership on an entity's Board of Directors or advisory committees; Megakaryon Co: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin Co., Ltd.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Eisai: Research Funding; DAIICHI SANKYO Co., Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; CHUGAI PHARMACEUTICAL Co., Ltd.: Honoraria; Bristol-Myers Squibb Co: Honoraria; Astellas Pharma Inc.: Consultancy, Honoraria; Amgen Astellas BioPharma: Honoraria; TEIJIN PHARMA LIMITED.: Honoraria. Uchida: Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Astellas Pharma Inc.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Novartis Pharma Inc.: Honoraria. Atsuta: Astellas Pharma Inc.: Speakers Bureau; Mochida Pharmaceutical Co., Ltd.: Speakers Bureau; AbbVie GK: Speakers Bureau; Kyowa Kirin Co., Ltd: Honoraria; Meiji Seika Pharma Co, Ltd.: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal